E – FLOC® Wastewater Solutions is proud to offer new, electrolytic disinfection technology for the destruction of bacteria, viruses and cysts as well as oxidation of VOC, H2S and other organic species. The process also applies for the oxidation / reduction of NH4, NO3 and TN without biological systems. This technology incorporates Dimensionally Stable Electrodes that do not sacrifice into solution and provide exponentially greater surface area than typical electrodes available on the market today. This technique is accomplished via the Reactive Electrolytic Membrane (REM), a newly developed, porous electrode fabricated from Magneli Phase, Titanium Oxide (Ti4O7) generating high concentrations of a variety of Mixed Oxidant species. The process is particularly useful for Nutrient Management in various food, meat processing and agricultural industries. Other applications include medical wastes, landfill leachate and semi-conductor wastewater. The following summary explains how these Mixed Oxidants are generated.
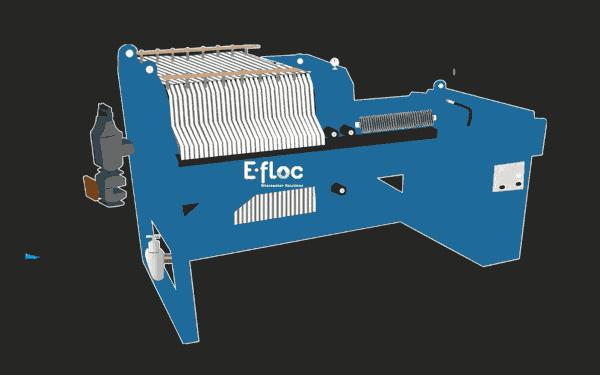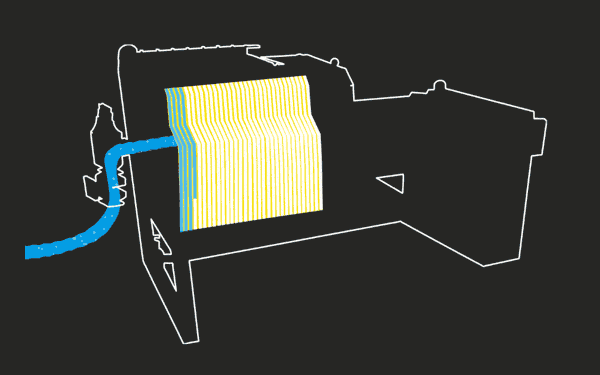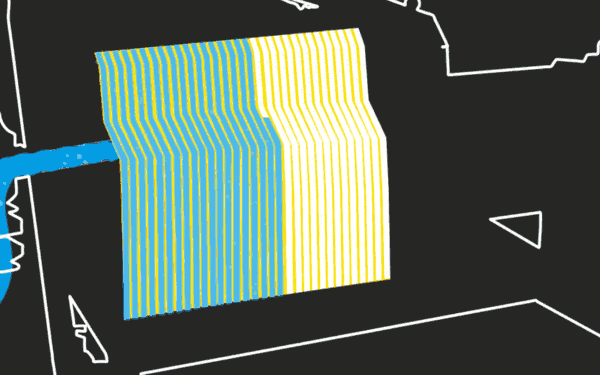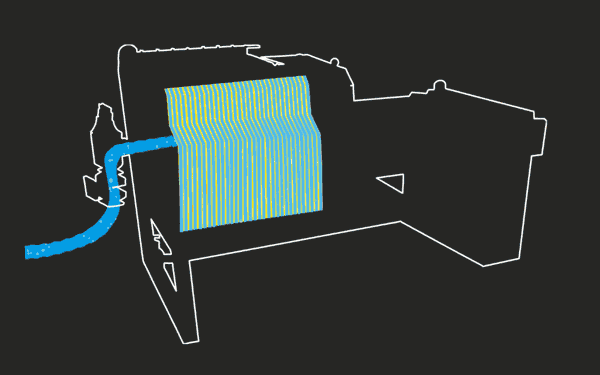
Reactions at the Cathode
Cathodic Reduction of Oxygen generates Active Oxygen Species:
1. Dissolved Oxygen is reduced to the Superoxide Ion.
O2 + e– → O2 ¯⁰
2. Superoxide Ion reacts with H+ to form the Peroxyl Radical.
O2-⁰ + H+ → HO2⁰
3. Peroxyl Radical degrades to Hydrogen Peroxide and Oxygen.
2 HO2⁰ → H2O2 + O2
4. Hydrogen Peroxide degrades to Hydroxyl Radical via two pathways.
a. Photolysis: H2O2 → 2 HO⁰
b. Reduction: H2O2 + e– → HO⁰ + HO–
Simultaneous Cathodic Reduction of water yields Hydrogen and Hydroxyl ion:
2 H2O + 2e– → H2↑ + 2 OH–


Reactions at the Anode
Anodic Oxidation of water yields Hydroxyl Radical:
H2O → HO⁰ + H+ + e–
Anodic Oxidation of chloride ion yields hypochlorite ion:
2 Cl– – 2e– → Cl2 ↑
Cl2↑ + 2 OH– → H2O + OCl– + Cl–
Dissolved Iron present in produced water reacts via the classic Fenton Reagent:
Fe+2 + H2O2 → Fe+3 + HO⁰ + OH–
Iron may also be Oxidized into solution from an Iron Anode to enhance Fenton Reagent:
Fe0 → Fe+2 + 2e–
There are other reactions occurring between the many dissolved constituents present in produced waters and leachate that are too numerous to be included in this discussion. Both organic and inorganic species react continuously throughout the process simultaneously, resulting in the precipitation of inorganics, oxidation of organics and the destruction of bacteria and algae. Due to the high TDS and corresponding high Electrical Conductivity of these waters, reactions occur at low DC Voltages and are very efficient and economical. A high concentration of Residual Mixed Oxidants remain to prevent the recontamination of water stored in ponds from bacteria and algae re-growth. These Mixed Oxidants persist when injected into the formation eliminating the need for expensive biocides and surfactants for disposal wells. Formation pressure is minimized and formation fouling is considerably reduced to minimize workover requirements and reduce overall disposal costs.
Contaminates Removed
- PFOS / PFOA (R & D)
- Ammonia (NH4)
- Nitrates (NO3)
- Total Nitrogen
- Biological Oxygen Demand (BOD)
- Chemical Oxygen Demand (COD)
- Total Organic Carbon (TOC)
- Soluble Hydrocarbons (BTEX/TPH/VOC)
- Bacteria, Viruses, Cysts
- Sulfides / H2S
Services:
- Mobile Treatment Systems & Services
- Turn-Key Design and Build Capabilities
- Installation / Commissioning / Operator Training
- Custom Engineering and Upgrades of Existing Equipment
- Treatability Studies / Field Pilot Studies / On-site Bench Studies
- Service and Maintenance Programs
- Rental / Lease / Service Options